Abstract
Background:
AML is a complex disease that encompasses a huge variation of cytogenetic and mutational backgrounds, which is often complicated by age related functional deficits (Klepin et al. 2013). Given the expanding availability of reduced-intensity treatment options, patient fitness and frailty measures have become increasingly instrumental in the decision between a wide range of treatment options. Furthermore, studies have also demonstrated potential benefits in older patients who receive intensive induction chemotherapy (Julisson 2011), creating a need for additional assessments to identify suitable induction candidates. Some frailty-associated assessments including timed-up-and-go test (TUGT) and the short physical performance battery have been linked to outcomes but are not yet in broad clinical use (Kleplin et al. 2013, Khalaf et al. 2020). To minimize the burden of implementing new patient assessments, we evaluated the utility of commonly available clinical measures of frailty-associated factors including sit-to-stand test and iADL status in predicting patient outcomes.
Methods:
We performed a retrospective cohort study of elderly patients newly diagnosed with AML at Juravinski Cancer Center between Jan 2019 and Dec 2020. We examined a total of 44 patients aged 65+. The primary outcome was overall survival (OS). Significant risk factors were identified using the Cox proportional hazards model.
Results:
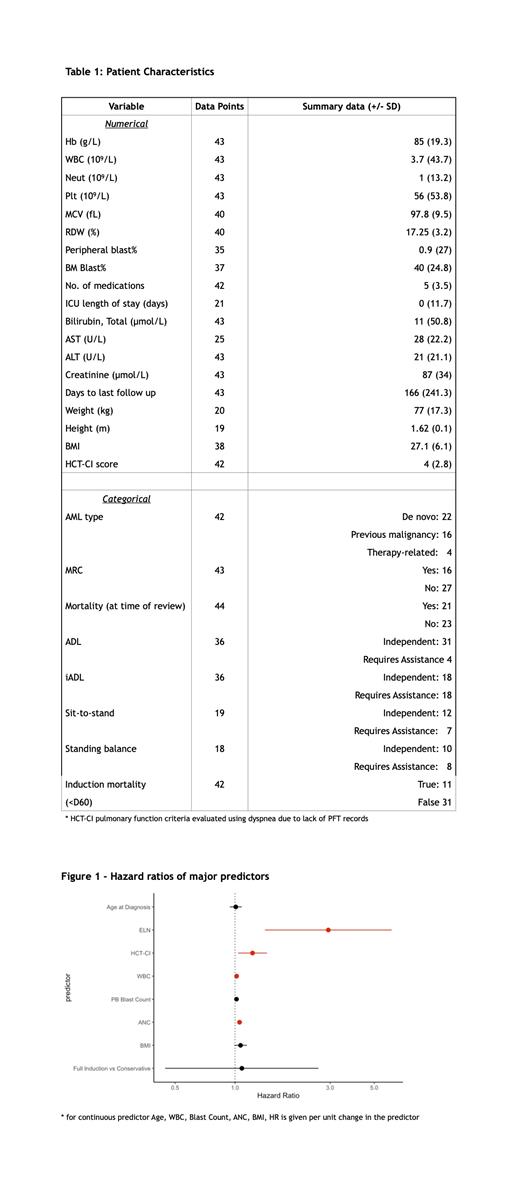
43 patients had sufficient data and were included in the analysis. The median age was 70 years (range 65-89), 53% were female and 47% were male. At the time of review, 21 patients were deceased (48.8%). The median survival time was 345 days. 26 patients received full induction treatment (7+3, FLAG-IDA, or Vyxeos), and 18 received conservative treatment (LDAC, azacytidine, or supportive care). The clinical wellness of the patients at diagnosis time was assessed by baseline clinical and laboratory findings. (Table 1) The Cox regression model was used to examine these variables in predicting overall survival (Figure 1). As expected, the ELN risk group was significantly correlated with OS (HR 2.94 95%CI [1.41-6.11]), along with laboratory measures of disease burden including blood blast count (HR 1.02 [1.00-1.03]), WBC, (HR 1.02 [1.01-1.03]) and ANC (HR 1.05 [1.02-1.08]).
We then assessed the influence of patient fitness factors on OS. The HCT-CI score was used as an aggregate comorbidities measure. While typically used for post-SCT prognosis, we found that HCT-CI assessed at diagnosis time was a significant predictor of OS (HR 1.22[1.04-1.45]). When added to a multivariate Cox model including ELN and age, the HCT-CI independently predicted OS (p = 0.003) and improved prediction efficiency by the Akaike information criteria (115 vs 122). This corroborates earlier findings by Sorror et al. 2017, who showed incorporation of biochemical and AML-specific variables to HCT-CI yields improved prognostic value. Within the HCT-CI, the AST and cardiac disease scores were the most associated with OS (HR 1.03[1.00-1.05] and 2.27[1.09-4.76]). While clinical and laboratory assessments were readily available, functional assessments of frailty were scarce. Previously reported frailty measures such as KPS score or TUGT were not assessed at the study center. OT/PT routinely administer sit-to-stand or standing balance tests as a part of fall risk assessment. However, this data was only available for 18 patients (41.8%). Independent sit-to-stand was not detected as a significant OS predictor (HR 0.63[0.14-2.83]), possibly related to the very limited sample size. BMI was marginally predictive (HR 1.07[0.99 - 1.08]), but unlike HCT-CI it was not an independent predictor when combined with ELN. Patient age did not significantly predict OS.
Conclusion:
This single center retrospective study was aimed at examining the role of existing clinical or functional measures of fitness and frailty in predicting overall survival. HCT-CI and ELN were found to be the most predictive factors amongst the variables examined, suggesting that a morbidity index such as HCT-CI could provide prognostic utility. However, functional assessments of frailty were not readily completed, limiting the ability to evaluate their usefulness. A future larger prospective study focused on optimizing and incorporating routine functional assessments of frailty is needed to address this topic.
Leber: Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Otsuka: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TaiHo: Honoraria, Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Khalaf: Novartis: Honoraria; Paladin: Honoraria; Pfizer: Honoraria.